Services
Wise Pharmacovigilance and Risk Management provides a range of services to help you meet your patient safety and regulatory PV requirements.
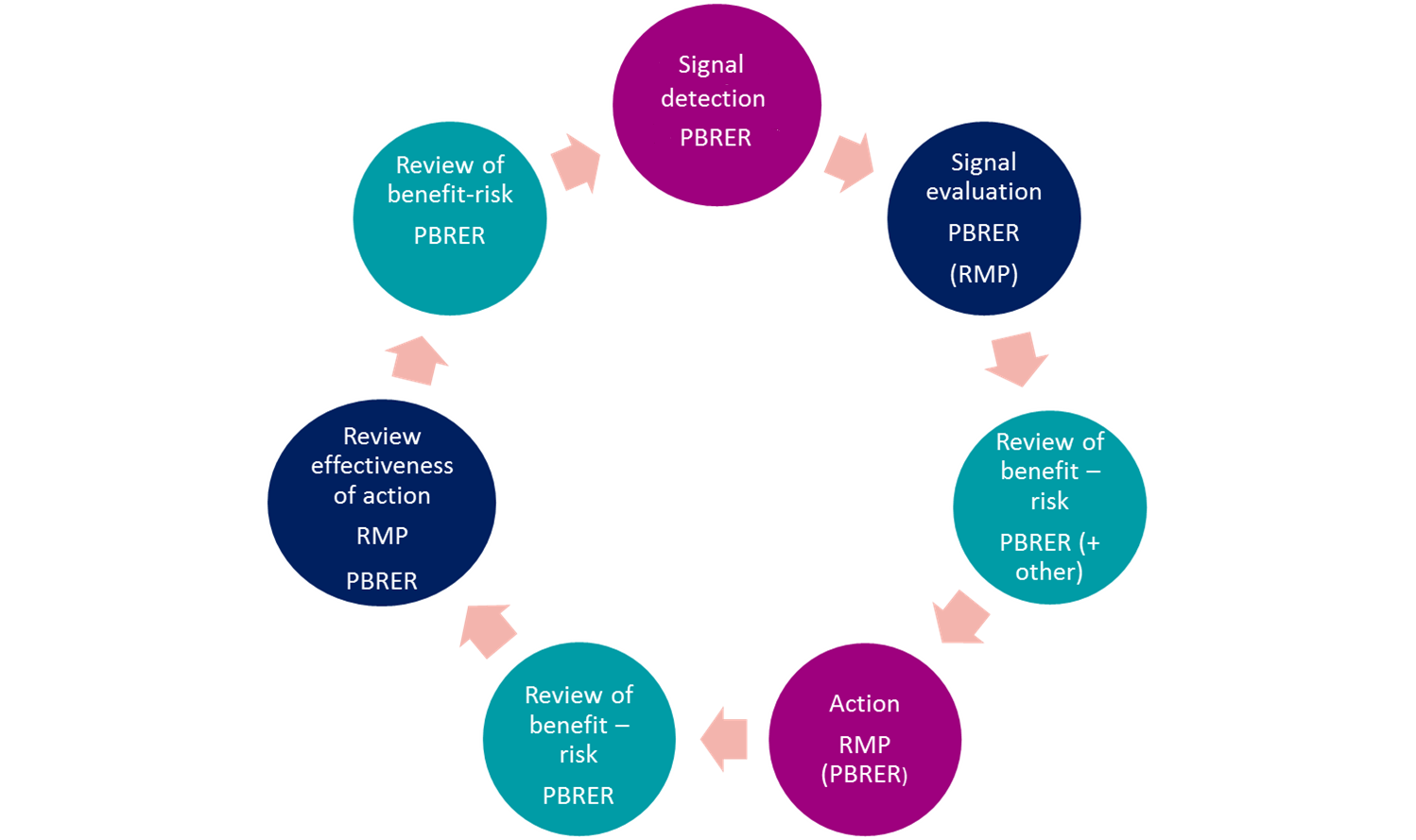
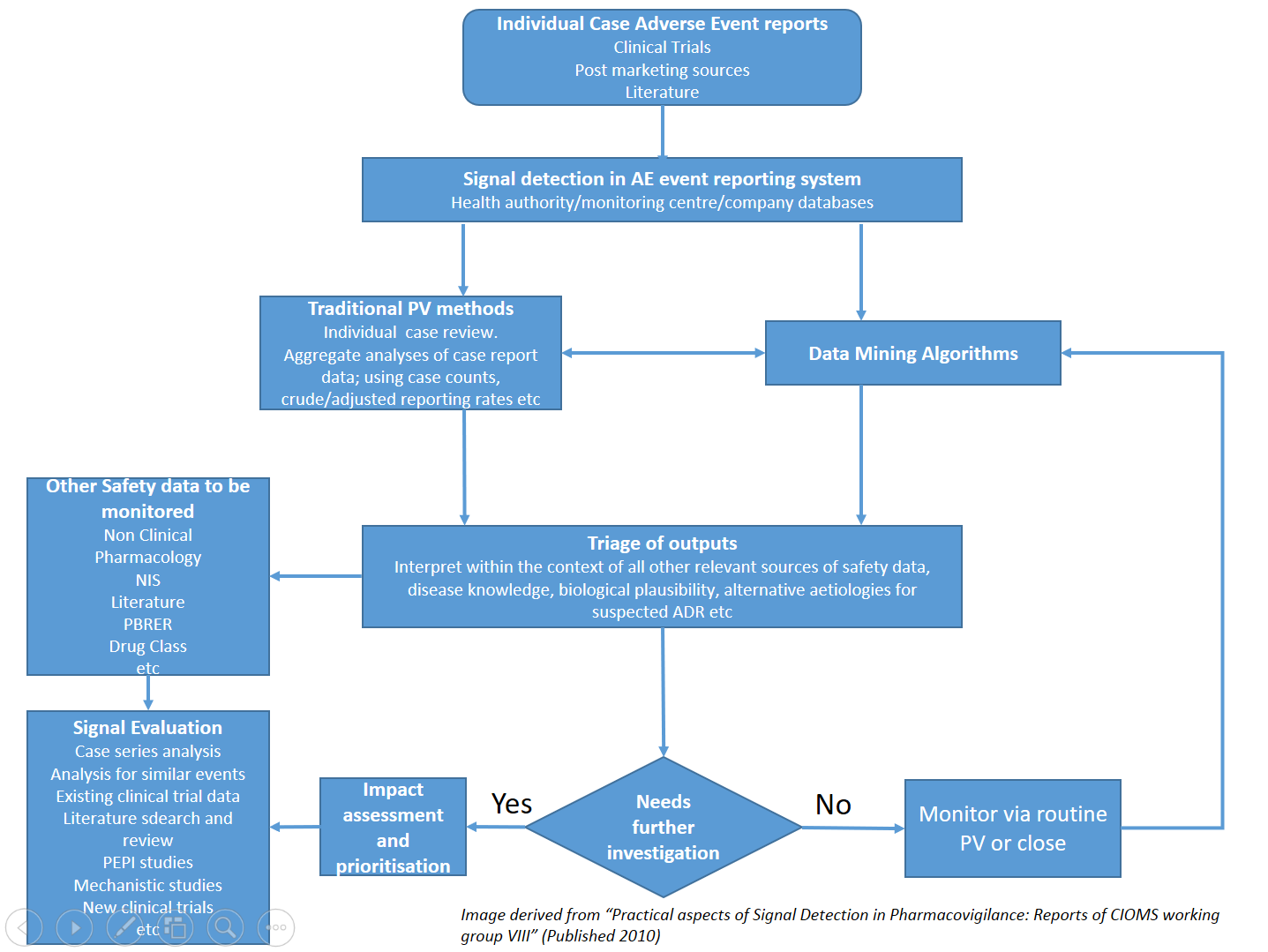
Signal management processes
- Ensure your signal detection and management processes meet global requirements
- Comply with regulations including Good Pharmacovigilance Practice (GVP) module IX
-
Create an holistic process to:
- quickly identify serious reactions that may impact patient safety
- incorporate data mining of regulatory databases
- review processes for company held data
- Ensure that your processes have an audit trail and are inspection ready
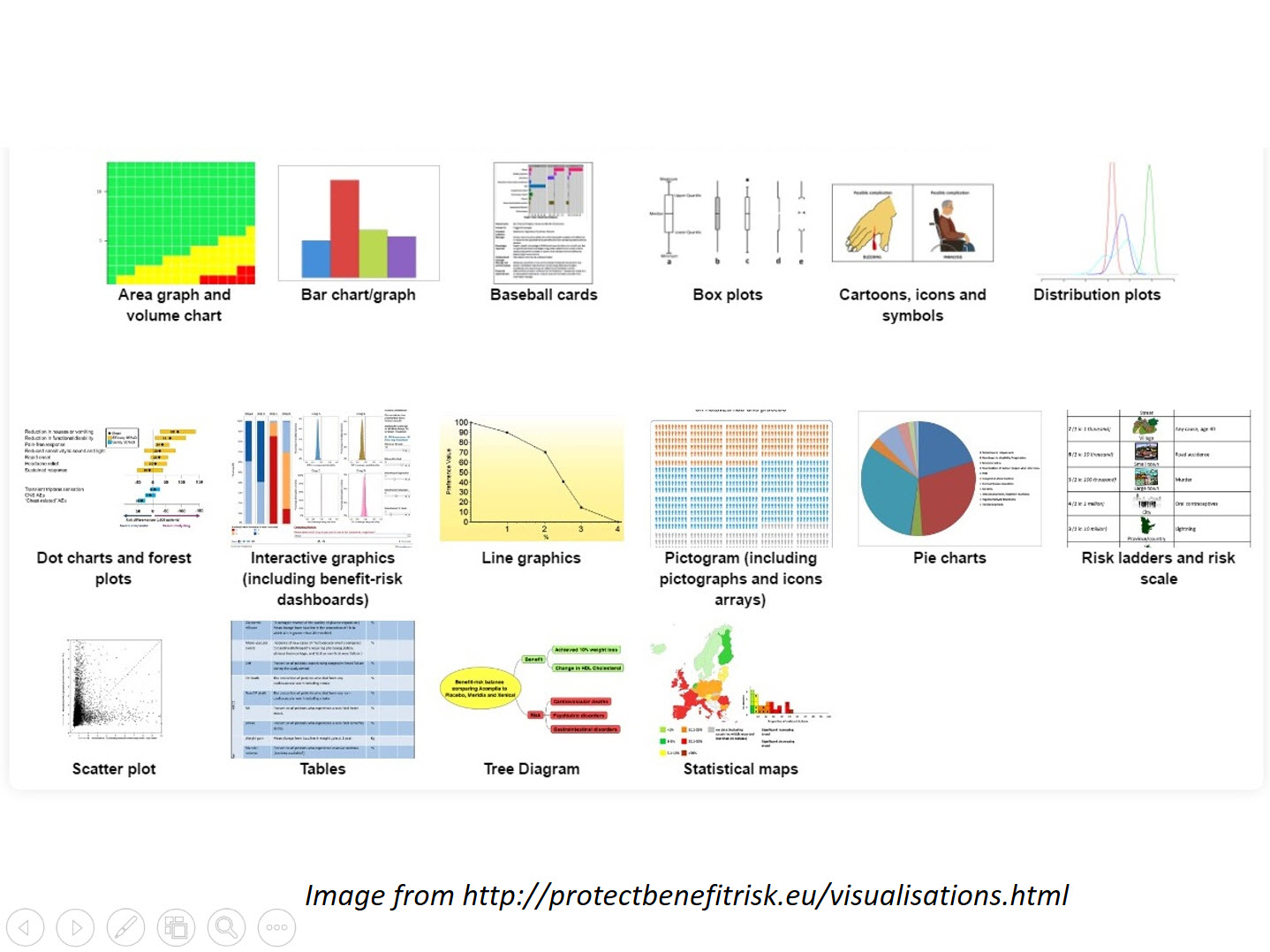
Benefit-risk Management
We can provide guidance on processes and write documents or sections of regulatory submissions including
- Implementation of ICH M4E(R2) in the CTD
- Benefit Risk presentation and discussion in aggregate reports such as DSUR and PBRER
Risk Management Planning
We provide design and implementation advice for risk management activities including
- Post approval safety studies
-
Risk Minimisation measures and evaluation studies
- REMS
- Global implementation approaches
Pharmacoepidemiology
Our in depth experience guides appropriate study design advice and protocol creation for post approval safety studies based on electronic health records or primary data collection. We will ensure compliance with
GVP VIII, ENCePP and GPP guidances
For clients who wish to engage our complete study execution services we can provide protocol creation, database study design, execution and report writing
Contact us to discuss your needs